H2O2 Decomposition Volumes
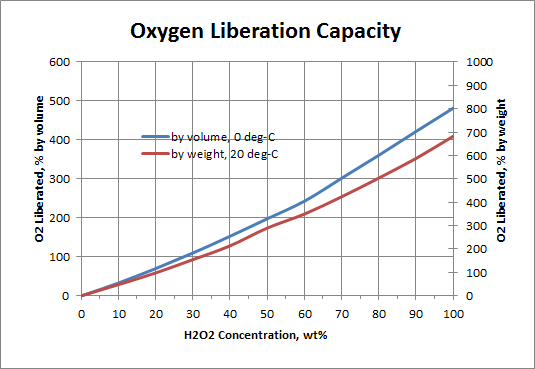
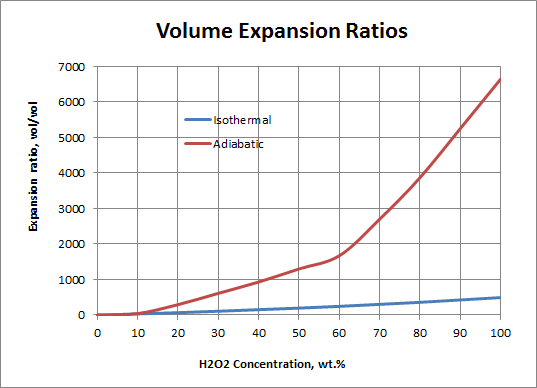
Notes: Isothermal refers to the slow, controlled decomposition where ambient temperature and pressure are maintained - the gas volume is comprised of essentially pure oxygen. Adiabatic refers to the rapid, accelerated decomposition where the temperature is allowed to increase but the pressure remains ambient - the gas volume is comprised of both oxygen and steam (water vapor).