Ultraviolet Absorption Spectrum
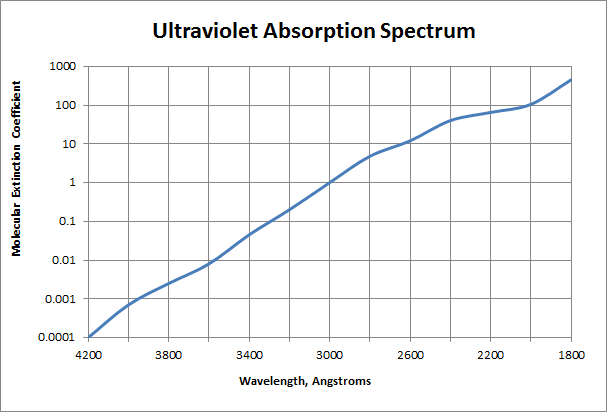
Molecular extinction coefficients for ultraviolet radiation for liquid and vapor H2O2 – water mixtures:
| Wavelength (Angstroms) | Molecular extinction coefficient ε, liters/mole.cm |
| 4000 | 0.00066 |
| 3800 | 0.0022 |
| 3600 | 0.01 |
| 3400 | 0.047 |
| 3200 | 0.22 |
| 3000 | 1 |
| 2800 | 4.2 |
| 2600 | 13 |
| 2537 | 19.6 ± 0.3 |
| 2400 | 35 |
| 2200 | 76 |
| 2000 | 140 |
Notes:
- The absorption coefficient for both liquid and vapor H2O2 is essentially the same.
- The shape of the curve relating the extinction coefficient to the wavelength is slightly parabolic.
- The absorption of ultraviolet radiation by H2O2 results in dissociation of the molecule into two hydroxyl radicals (HO.), although other reactions are possible and may occur to some extent.
- Beer’s law is not strictly obeyed by H2O2 solutions, as higher concentrations of H2O2 absorb to a greater extent than Beer’s law would predict (i.e., the molecular extinction coefficient decreases as H2O2 concentration increases > 50% wt.%).
- The presence of alkali shifts the absorption curve toward the visible (i.e., increases the absorption coefficient). This is due to the dissociation of H2O2 into the perhydroxyl ion (HO2-) that absorbs more intensely than H2O2.