H2O2 Refractive Index
Note: Care must be taken to ensure that the sample holder or prism is non-catalytic to H2O2 lest the oxygen bubbles formed through decomposition interfere with the measurements.
The refractive index for H2O2 is greater than that of water, and the curve relating to composition is slightly concave upward.
Experimental values for refractive index:
| H2O2 Conc.wt.% | ηD |
|---|
| 25 °C | 20 °C |
|---|
| 0.00 | 1.33251 | 1.33299 |
| 10.10 | 1.33881 | 1.33946 |
| 19.98 | 1.34521 | 1.34603 |
| 30.11 | 1.35203 | 1.35296 |
| 40.03 | 1.35885 | 1.35986 |
| 50.10 | 1.36611 | 1.36724 |
| 60.66 | 1.37389 | 1.37508 |
| 70.15 | 1.38151 | 1.38284 |
| 79.86 | 1.38927 | 1.39072 |
| 92.36 | 1.39998 | 1.40157 |
| 96.26 | 1.40333 | 1.40495 |
| 99.30 | 1.40607 | 1.40774 |
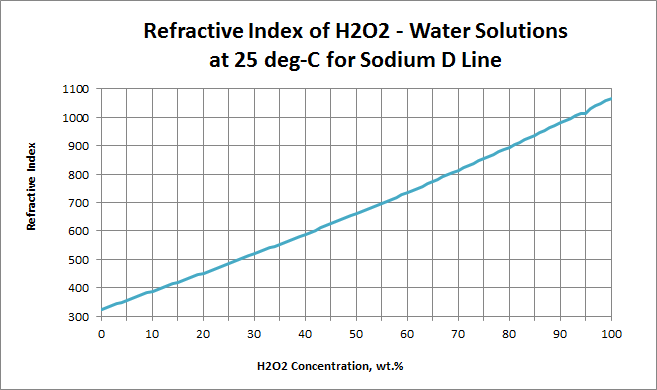
Ref: P.A. Giguere and P.Geoffrion, Can. J. Res., 27B:168 (1949)
The temperature coefficient for the refractive index is also greater for H2O2 than it is for water: 105 αn = 7.7 + 16.5 ω
Temperature corrections to be subtracted from the percentage of H2O2 determined above:
Temp.
°C | Approximate Concentration, wt.% |
|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|
| 20 | 0.9 | 1.2 | 1.3 | 1.4 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 |
|---|
| 21 | 0.8 | 1.9 | 1 | 1.1 | 1.1 | 1.2 | 1.2 | 1.3 | 1.4 | 1.5 |
|---|
| 22 | 0.6 | 0.7 | 0.8 | 0.9 | 0.9 | 1 | 1 | 1 | 1.1 | 1.1 |
|---|
| 23 | 0.4 | 0.4 | 0.5 | 0.6 | 0.6 | 0.6 | 0.6 | 0.7 | 0.7 | 0.8 |
|---|
| 24 | 0.2 | 0.2 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.4 | 0.4 |
|---|
Ref: P.A. Giguere and P.Geoffrion, Can. J. Res., 27B:168 (1949)
Constants derived from refractive data:
| | H2O2 | H2O |
| Specific Refraction | rD | cc/g, 25 °C | 0.1705 | 0.206 |
|---|
| Molar Refraction | [R]D | cc/mole, 25 °C | 5.801 | 3.712 |
|---|
| Polarizability | α x 1024 | cc/molecule, 25 °C | 2.3 | 1.47 |
|---|
| Molar Dispersion | [R]G - [R]c | cc/mole, 20 °C | 1.3576 | 0.929 |
|---|
| Dispersion Constant | α x 10-30 | sec-2, 20 °C | 8.479 | 6.532 |
|---|
| Characteristic Frequency | ν 0 x 10-15 | sec-1, 20 °C | 2.979 | 2.94 |
|---|
Ref: P.A. Giguere, Can. J. Res. 21B:156 (1943)
P.A. Giguere and P.Geoffrion, Can. J. Res., 27B:168 (1949)