Peroxidase Enzyme Catalyzer
Principle
Peroxidase enzyme catalyzes the transfer of electrons from H2O2 to a colorimetric indicator. The absorbance of 596 nm light by the sample is compared to a reference curve generated by standard H2O2 solutions.
Scope of Application
New applications for H2O2 within the food processing and drinking water industries require the accurate measurement of residual H2O2 to 0.1 mg/L. This method is suitable for these and similar applications where the water matrix is clear and free of turbidity. Samples containing greater than 0.2 mg/L may be diluted with distilled water to a concentration suitable for this method.
Interferences
The peroxidase-H2O2 reaction is highly selective and not typically subject to interferences. However, excessive turbidity or any contaminate that absorbs ultraviolet light at 596 nm may impact the accuracy and sensitivity of the method.
Safety Precautions
Concentrated sulfuric acid is a corrosive, hazardous material and should be handled and disposed of in accordance with the MSDS. Neoprene gloves and monogoggles are recommended, as is working under a vacuum hood.
Sample bottles containing H2O2 should not be stoppered, but rather vented or covered loosely with aluminum foil or paraffin film.
Reagents
- Peroxidase (Type II 190 purpurogallin units/mg, Sigma Chemical Co., St. Louis, MO). Dissolve 10 mg of peroxidase in 10 mLs of distilled water.
- Leuco crystal violet (Aldrich Chemical Co.). Dissolve 50 mg of leuco crystal violet in 100 mLs of 0.5% HCl solution.
- Acetate buffer solution pH 4.5. Mix equal volumes of 2 M sodium acetate and 2 M acetic acid and adjust the pH to 4.5 with glacial acetic acid.
- Hydrogen peroxide solution. Prepare a 3,000 mg/L (0.3%) stock solution of H2O2 by diluting 35 or 50% H2O2 with distilled water. Standardize the 3,000 mg/L solution with 0.1 N KMnO4 and use this solution to prepare a 1.5 mg/L H2O2 calibration solution.
Apparatus
Varian Model 634 UV-Visible Spectrophotometer or equivalent with matched cells of 1cm.
Procedure
General - It is important that all testing equipment be either clean plastic (e.g., polycarbonate or polyethylene or passivated glassware). Glassware can be passivated by soaking clean glassware in 10% nitric acid for four (4) hours at 70 deg-C and then twenty (20) hours at room temperature. The glassware is then rinsed with distilled water and oven dried at 110 deg-C. Passivated glassware should be stored by covering the opening with aluminum foil.
-
Standardization of H2O2 Stock Solution
The 3,000 mg/L stock solution of hydrogen peroxide is standardized before use with 0.1 N KMnO4 Pipet 10 mLs of the solution into a beaker, add 5 mLs of 20% H2SO4 and titrate with 0.1 N KMnO4 until the pink color of KMnO4 appears in the solution.
mg/L H2O2 = (mLs of 0.1 N KMnO4) x 170.1
Wt. % H2O2 = (mLs of 0.1 N KMnO4) x 0.017
-
Calibration Curve
- Add 0.11 mL of the 1.5 mg/L H2O2 calibration solution to 0.89 mL of distilled water in a 20 mL vial. Add successively 1 mL of the leuco crystal violet solution, 0.5 mL of the peroxidase solution, and 6 mLs of the acetate buffer solution. This solution has a H2O2 concentration of 0.01 mg/L.
- Mix the solution gently and wait five minutes for color development.
- Place the solution in a 1 cm quartz cell and measure the absorbance at 596 nm versus a blank solution in a matched cell.
- Repeat (a) with 0.33 mL of the diluted 1.5 mg/L H2O2 solution and 9.67 mLs of distilled water; 0.55 mL of the diluted H2O2 solution and 9.45 mLs of distilled water; 1.1 mL of the diluted H2O2 solution and 8.9 mLs of distilled water; and 2.2 mLs of the diluted H2O2 solution and 7.8 mLs of distilled water. These solutions have H2O2 concentrations of 0.03, 0.05, 0.1 and 0.2 mg/L, respectively.
-
Plot the absorbance against the concentration and draw the best straight lime through the experimental points. A sample curve is shown below.
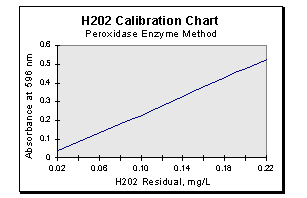
-
Sample Analysis
- For a sample containing about 0.1 mg/L H2O2 - add 10 mLs of this solution into a 20 mL vial, add successively 1 mL of the leuco crystal violet solution, 0.5 mL of the peroxidase solution, and 5 mLs of the acetate buffer. Mix the solution gently and wait five minutes for color development.
- Place the solution in a 1 cm quartz call and measure the absorbance at 596 nm versus a blank reagent solution in a matched cell.
- Obtain the absorbance and read the corresponding concentration of H2O2 from the calibration curve.
- The H2O2 concentration determined from the curve should be multiplied by a dilution factor of eight-fifths (1.6) to give the concentration of the sample.
References
Chin, H.S. and Cortes, A., "Determination of Hydrogen Peroxide: A Comparison Between the Potentiometric Titration Method and an Enzyme Catalyzed Procedure", Unpublished Draft, National Food Processors Assn., 1950 Sixth Street, Berkeley, CA 94710, 1982.