Cobalt Bicarbonate Method
Principle
H2O2 reacts with cobalt ion to produce a colored peroxo-cobalt complex. The absorbance of 260 nm light by the sample is compared to a reference curve generated by standard H2O2 solutions.
Scope of Application
New applications for H2O2 within the food processing and drinking water industries require the accurate measurement of residual H2O2 to 0.1 mg/L. This method is suitable for these and similar applications where the water matrix is clear and free of turbidity. Samples containing greater than 0.2 mg/L may be diluted with distilled water to a concentration suitable for this method.
Interferences
Reducing agents such as bisulfite (that may be slow to react with free H2O2) may quickly react with the Co-H2O2 complex to provide false negative readings. Also, any contaminate that absorbs ultraviolet light at 260 nm may impact the accuracy and sensitivity of the method.
Safety Precautions
Cobalt salts are persistent contaminants and should not be released into the environment. Spent solutions should be collected and disposed of in an approved manner.
Concentrated sulfuric acid is a corrosive, hazardous material and should be handled and disposed of in accordance with the MSDS. Neoprene gloves and monogoggles are recommended, as is working under a vacuum hood.
Sample bottles containing H2O2 should not be stoppered, but rather vented or covered loosely with aluminum foil or paraffin film.
Reagents
- Cobalt (II) solution. Dissolve 19 gms of CoSO4:7H2O in one liter of distilled water.
- Saturated sodium bicarbonate solution. (solubility in cold water is about 100 g/liter).
- Sodium hexametaphosphate solution. Dissolve 10 gms of (NaPO3)6 in one liter of distilled water.
- 0.1 N potassium permanganate solution (Fisher certified concentrate).
- Hydrogen peroxide solution. Prepare a 3,000 ppm (0.3%) stock solution of H2O2 by diluting 35 or 50% H2O2 with distilled water. Standardize the 3,000 ppm solution with 0.1 N KMnO4 and use this solution to prepare a 1.5 mg/L H2O2 calibration solution.
Apparatus
Varian Model 634 UV-Visible Spectrophotometer or equivalent with matched calls of 5 cm.
Procedure
General - It is important that all testing equipment be either clean plastic (e.g., polycarbonate or polyethylene) or passivated glassware. Glassware can be passivated by soaking clean glassware in 10% nitric acid for four (4) hours at 70 deg-C and then twenty (20) hours at room temperature. The glassware is then rinsed with distilled water and oven dried at 110 deg-C. Passivated glassware should be stored by covering the opening with aluminum foil.
-
Standardization of H2O2 Stock Solution
The 3,000 mg/L stock solution of hydrogen peroxide is standardized before use with 0.1 N KMnO4 Pipet 10 mLs of the solution into a beaker, add 5 mLs of 20% H2SO4 and titrate with 0.1 N KMnO4 until the pink color of KMnO4 appears in the solution.
mg/L H2O2 = (mLs of 0.1 N KMnO4) x 170.1
Wt. % H2O2 = (mLs of 0.1 N KMnO4) x 0.017
-
Calibration Curve
- Add 1 mL of the 1.5 mg/L H2O2 calibration solution to 79 mLs of distilled water in a 100 mL volumetric flask. Add successively 1 mL of the sodium hexametaphosphate solution and 1 mL of the Cobalt Solution, and make up the mixture to 100 mL with the saturated sodium bicarbonate solution. This solution has a H2O2 concentration of 0.015 mg/L.
- Lace the solution prepared in (a) into a 5 cm quartz cell and measure the absorbance at 260 nm versus a blank reagent solution in a matched cell.
- Repeat (a) with 2 mLs of the diluted 1.5 mg/L H2O2 and 78 mLs of the distilled water; 3 mLs of the diluted H2O2 and 77 mLs of the distilled water; 6 mLs of the diluted H2O2 and 74 mLs of the distilled water; and 12 mLs of the diluted H2O2 and 68 mLs of the distilled water. These solutions have H2O2 concentrations of 0.03, 0.043, 0.090 and 0.18 ppm, respectively.
- Plot the absorbance against the concentration. Draw the best straight line through the experimental points. A sample curve is shown in Figure 1 (below).
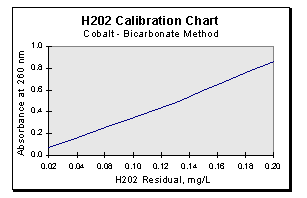
-
Sample Analysis
- For a sample containing about 0.1 mg/L H2O2, pipet 80 mLs into a 100 mL volumetric flask. Add successively 1 mL of the sodium hexametaphosphate solution and 1 mL of the cobalt solution, and make up the mixture to 100 mLs with the saturated sodium bicarbonate solution.
- Place the solution prepared in (a) into a 5 cm quartz cell and measure the absorbance at 260 nm versus a blank reagent solution in a matched cell.
- Obtain the absorbance and read the corresponding concentration of H2O2 from the calibration curve.
- The H2O2 concentration determined from the curve should be multiplied by a dilution factor five-fourths (1.25) to give the H2O2 concentration of the sample.
References
Masschelen, W., "Spectrophotometric Determination of Residual Hydrogen Peroxide", Water and Sewerage Works, p.69, August 1977.